| NOTE: Booklet available in Microsoft Word format, RTF format and PDF. Published by Rodale Press, Inc., 1980. A project of Western SUN Arizona and Western Solar Utilization Network. |
Introduction to Solar Energy
The sun's energy arrives on earth in the primary form of heat and light. Other aspects of solar radiation are less easily perceived and their detection often requires sophisticated equipment. All solar radiation travels through space in waves, and it is the length of these waves (the shortest is less than a millionth of an inch, the longest more than a thousand yards) by which all solar radiation is classified. The aggregate of all radiation aspects of the sun is called the solar spectrum.
There are two important facets about the solar spectrum.
1. While the sun emits radiation in all wavelengths, it is the short wavelength radiation that accounts for the majority of energy in the solar spectrum. For example, the portion of the spectrum perceived as the visible light is a relatively small segment compared to the variety of spectrum wavelengths, yet accounts for 46 percent of the energy radiating from the sun. Another 49 percent, that which is perceived as heat, is derived from the infrared band of the spectrum.
2. The proportion of different wavelengths in the solar spectrum does not change and therefore the energy output of the sun remains constant. A measurement of this phenomena is known as the Solar Constant, defined as the amount of heat energy delivered by solar radiation to a square foot of material set perpendicular to the sun’s rays for one hour at the outer edge of the earth’s atmosphere. The Solar Constant measurement is about 429.2 BTU’s with minimal changes over the year. The energy measured as the Solar Constant is not a measure of the amount of solar energy that actually reaches the earth’s surface, since as much as 35 percent of all the solar radiation intercepted by the earth and its surrounding atmosphere is reflected back into space. Additionally, water vapor and atmospheric gases absorb another 15 percent. As a global average only about 35-40 percent of the solar radiation entering the atmosphere actually reaches the earth’s surface.
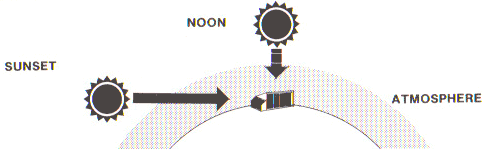
As a practical matter, global averages are of little interest. The essential point is that the atmosphere impacts on the amount of solar energy that actually reaches the earth’s surface - the more atmosphere solar radiation has to move through, the more is lost on the way. In this regard, two celestial events – the daily rotation of the earth and its seasonal tilt of the earth's axis – are important in determining the length of atmosphere through which the sun’s rays must pass before striking any particular location on the globe (Fig. 1).
|
|
| Figure 1. The amount of solar energy reaching the earth's surface is determined by the amount of atmosphere through which it must pass. |
These events set the upper limit amount of solar energy that can reach the surface of the earth at any location on any day of the year.
ENERGY DENSITY
One of the conditions for accurately measuring the Solar Constant requires the intercepting surface to be perpendicular to the sun’s rays. Since solar radiation travels in parallel rays, the perpendicular position identifies the maximum density of rays striking a surface. Any deviation from perpendicular reduces the radiation density and the amount of energy intercepted. This is best illustrated in Fig. 2.
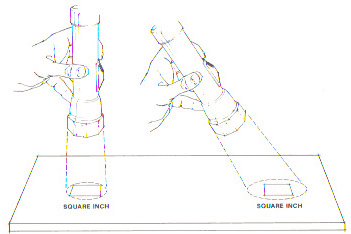 Figure 2. These illustrations demonstrate how energy density is determined by the angle of incidence. The amount of light emitted by the flashlight is the same in both illustrations but it is spread over a larger area (right) when the light is tilted away from its original perpendicular position (left). Figure 2. These illustrations demonstrate how energy density is determined by the angle of incidence. The amount of light emitted by the flashlight is the same in both illustrations but it is spread over a larger area (right) when the light is tilted away from its original perpendicular position (left). |
The angle created by incoming radiation and a line perpendicular to an intercepting surface is called the angle of incidence. Table 1 illustrates that a fairly large increase in the angle of incidence results in only a modest reduction in intercepted radiation.
RADIATION AND SURFACES
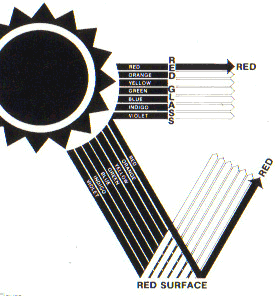
When sunlight strikes a surface it is reflected, transmitted or absorbed, in any combination depending on the texture, color and clarity of the surface. All completely opaque surfaces both reflect and absorb radiation but do so in different ways. For example, a rough surface such as stucco reflects sunlight in a scattered fashion while a smooth, glossy surface reflects uniformly and at an angle equal to the angle of incidence. The wavelengths of solar radiation that are reflected are determined by the color of the surface material. A red stucco surface, for example, will scatter (diffuse) wavelengths in the red band of the spectrum and absorb all others (Fig. 3), while a white glossy surface will reflect all wavelengths in the visible spectrum at an angle equal and opposite to the angle of incidence.
|
|
| Figure 3. Color is perceived when visible light is reflected from a surface. Red surfaces reflect red wavelengths and absorb all others. |
Conversely, a rough black surface absorbs all wavelengths in the visible spectrum, while the transparent surface of window glass allows nearly all radiation to pass through it with comparatively little reflection or absorption, and without deflecting it from its parallel lines of travel. Translucent materials also transmit radiation but scatter the rays as they pass. It should be noted that relatively few materials are excellent reflectors, transmitters, or absorbers of the sun’s rays.
HEAT BEHAVIOR:
HEAT ABSORPTION
Sunlight, in the form of short wave solar radiation, exhibits a transformation from solar energy to heat energy when impacting a material (absorption). The temperatures of a white surface and a black surface exposed the same direct sunlight is a simple demonstration of this conversion. The temperature of the black surface is higher because it is absorbing more solar energy. As solar energy is absorbed at the surface of a material it stimulates movement of the molecules in the material. Molecular movement is measured in terms of heat – the greater the movement, the greater the heat. Since the color black absorbs more of the spectrum than the color white, it will in turn be hotter (more molecular excitement) than white.
CONDUCTION
As a material absorbs radiation and molecular movement continues to accelerate, the heat energy is redistributed through the material due to the natural phenomenon of maintaining equilibrium. This occurs when stimulated molecules, vibrating at a faster rate, impact adjacent molecules vibrating at a slower rate, thereby dissipating and "spreading the wealth". In this way, heat is conducted away from the source of energy as the material seeks to distribute the energy evenly throughout its mass. The rate at which energy flows or is conducted though a material depends on the density of the material and conduction, the rate at which molecules are capable of receiving and passing on energy. Gases are poor conductors; metals are comparatively good conductors; and less dense materials containing tiny air pockets and voids conduct heat at a much slower rate.
HEAT TRANSFER
Heat transfer from a solid material to a fluid medium (liquid or air) occurs by radiation (infrared). It is a continuation distributed molecular "bumping" between a solid material and a transfer medium (air or liquid). The added dimension of using fluids is they can move across a hot solid surface, allowing molecules of the fluid to become agitated (heat), then move away from the heat source, and t be replaced by new, unheated molecules. This process of fluid movement is called natural convection when the movement is unaided by machinery (i.e. hot air rises), and forced convection if the process is aided by a pump or fan.
The process occurs naturally as the molecules of a fluid begin to vibrate when heat is applied, and then becomes less dense (lighter) than the surrounding unheated fluid. The lighter heated molecules rise at a rate determined by the amount of heat applied. Boiling water is a good example of heated molecules near the burner rising quickly to the surface to the point of surface disruption (boiling). Steam generated by the process is simply water molecules whose vibration rate is violent enough to allow them to break from of the water surface.
Birds that seem to hang in the air without beating their wings are evidence of the power of natural convection. On clear sunny mornings, air at the surface of the ground (especially dark surfaces) is heated rapidly and rises in columns with enough force to suspend the bird overhead and even push it upward. The reverse of this process occurs as convected molecules get further from the heat source of heat, give up their energy (slower molecular excitement), and fall. Conduction and convection can be thought of as processes by which solar energy can be both transported and stored.
EMISSIVITY
The principle of solar energy absorption was discussed in terms of two surfaces exposed to the sun. Conduction was then discussed to show how absorbed solar energy moves through a material, always in a direction away from the source of heat to attain equilibrium. NOTE: Any molecular movement is continually generating heat in the form of radiant energy. Unlike solar energy, radiant energy is limited to infrared radiation emitted from a material at low temperatures. The extent to which a material emits thermal energy depends both on the temperature of the material and nature of its surface. Polished metal surfaces are poor emitters and poor absorbers of thermal energy. Again, as with solar radiation, the amount of thermal energy a surface will intercept depends on the angle of the incoming radiation.
Glass has the special characteristic of transmitting nearly all solar radiation that it intercepts (which moves through it) and is less transparent to most thermal radiation. The temperature build-up in a closed car on a sunny but cold day is evidence of this characteristic. Solar energy passes through the windows is absorbed by interior materials, and reradiated into the interior space in the form of thermal energy (heat) which is unable to pass back through the glass to the outside. This has become known as the greenhouse effect.
HEAT STORAGE
All materials can store heat to some degree. The ability of a material to do so is called its specific heat – the amount of heat, measured in BTU’s for a given mass, a material can hold when its temperature is raised one degree Fahrenheit. As an indicator of a material's value as a heat storage medium in solar heating of spaces, the specific heat of a material is not very useful. The usefulness of a material in such an application is determined by its heat capacity, a measurement of the specific heat of a material multiplied by its density. The higher the heat capacity, the more effective the material is for heating and cooling.
Finally, a good storage medium material must absorb heat when it is available, and give I t up when it is needed, and it must be a relatively good heat conductor. In Table 1 the comparative specific heat and heat capacity measurements for a variety of materials is given, and it shows there is no perfect storage medium in terms of volume, storage capacity, and conductivity.
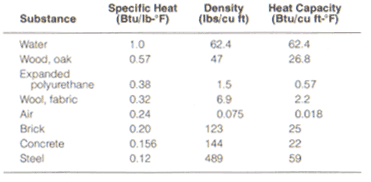 |
| Table 1. Specific heat and heat capacity of various surfaces. |
Next Part: Passive Solar Heating & Cooling Manual, Part 2 of 4 - Passive Solar Architecture - Heating